IFCC is pleased to announce that Proceedings of the 15th Bergmeyer conference, held in March this year, an IFCC Master Discussion sponsored by IFCC and Roche, are now available for free downloading.
Listado de emisiones anteriores
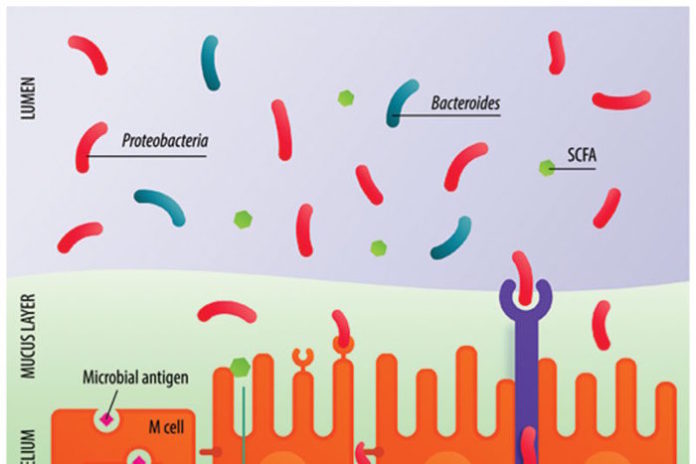
Mitochondrial DNA levels as a marker of embryo viability in IVF
Genetic screening techniques so far have relied largely on the assessment of one component of the embryo’s genetic constitution, the number of chromosomes in its cells. Studies dating back 20 years have shown beyond doubt that chromosomal abnormality is common in preimplantation embryos, and becomes even more common with increasing age. Chromosomal anomalies – or aneuploidy – are universally accepted as the main reason for miscarriage and the main cause of implantation failure
Methods that allow the screening of IVF embryos for aneuploidy are increasingly used during fertility treatments, helping doctors ensure that the embryos transferred have the correct number of chromosomes. However, even when a chromosomally normal embryo is transferred about one-third fail to produce a pregnancy.
Now, a new approach to embryo assessment described at this year’s Annual Meeting of ESHRE may be able to shed light on why so many apparently healthy embryos are not viable. The approach is based on the quantification of mitochondrial DNA found in the outermost layer of cells in a five-day old embryo. The combination of chromosome analysis and mitochondrial assessment may now represent the most accurate and predictive measure of embryo viability with great potential for improving IVF outcome.
Following the presentation of these important results here in Helsinki, first author Dr Epida Fragouli from Reprogenetics UK and the University of Oxford’s Nuffield Department of Obstetrics and Gynaecology in Oxford, UK, said the study “demonstrates that mitochondrial DNA levels are highly predictive of an embryo’s implantation potential”. Even embryos which are chromosomally normal and have a good morphological appearance under the microscope, she added, have virtually no ability to produce a baby if they have unusually high levels of mitochondrial DNA.
The evidence for mitochondrial DNA as an accurate marker of embryo viability came in a prospective study of 280 blastocysts (embryos cultured for five or six days) and tested to be chromosomally normal. The study was the first ever evaluation of the predictive power of mitochondrial DNA quantification with a prospective, blinded, non-selection design. This meant that the mitochondrial DNA levels of the blastocysts were not known at the time of transfer, so study results relied solely on a comparison of IVF outcome and mitochondrial DNA level, and were not subject to any bias.
Of the 111 single blastocyst transfers whose outcome was so far known, 78 (70%) led to ongoing pregnancies, and every single one of them (100%) had levels of mitochondrial DNA known to be normal. The remaining 33 blastocysts failed to implant, and eight of these (24%) had unusually high levels of mitochondrial DNA. Stratifying IVF outcome with mitochondrial DNA levels of low, normal and high produced an ongoing pregnancy rate of 76% (78/103) for morphologically good chromosomally-normal blastocysts with normal levels of mitochondrial DNA, but of 0% (0/8) pregnancy rate for the same type of blastocysts but with unusually high mitochondrial DNA levels. This difference, said Dr Fragouli, was highly statistically significant (P<0.0001).
“The results confirm that embryos with elevated levels of mitochondrial DNA rarely implant,” she added, “and support the use of mitochondrial quantification as a marker of embryo viability.”
Dr Fragouli described the results as “very robust”, noting that the methodology has been extensively validated. This was done in a retrospective analysis of samples biopsied from over 700 blastocysts generated in multiple clinics in Europe and the USA, which confirmed, first, that blastocysts with unusually high mitochondrial DNA levels have greatly reduced implantation ability, and second, that a threshold of viability dependent on mitochondrial DNA levels was valid.
Dr Fragouli explained that mitochondrial DNA levels can be simply and quickly measured by a polymerase chain reaction (PCR) strategy, but next generation sequencing (NGS) can also be used. She also emphasised that all embryos must first be screened for aneuploidy and that the mitochondrial DNA test is only applicable to chromosomally normal embryos. “Aneuploidy is still the biggest cause of embryo implantation failure,” she explained, “so mitochondrial analysis does not replace that. It is the combination of the two methods – mitochondrial DNA testing and chromosome analysis – that are so powerful.” Mitochondrial DNA testing would add around £200 to the cost of aneuploidy screening. However, her group is working on an approach which would assess chromosome content and mitochondrial DNA simultaneously. “Once these are ready for application,” she said, “there would be no extra cost added.”
The group has started offering mitochondrial DNA quantification clinically in the USA, and has applied to the HFEA for a license for use in the UK.
Source: Eurekalert
Saliva: The Next Frontier in Cancer Diagnostics?
As momentum grows for implementing liquid biopsy in clinical practice, the technology for detecting cancer DNA circulating throughout the body is advancing on another front: saliva as the biopsy medium. Saliva offers a noninvasive alternative to blood draws, with testing potentially performed at the point of care and as sensitive and specific as other platforms. But challenges remain in finding a clinical niche for saliva liquid biopsy in an increasingly competitive field of more mature blood-based technologies.
Considerable research exploring saliva liquid biopsy has taken place at the University of California, Los Angeles (UCLA) School of Dentistry, under the watchful eye of David Wong, DMD, DMS, Felix and Mildred Yip professor and associate dean for research. From early funding in 2002 by the National Institute of Dental and Craniofacial Research, Wong’s lab has been developing iterations of saliva-based diagnostics, most recently with a self-contained desktop device called Electric Field Induced Released and Measurement (EFIRM).
Wong recently presented clinical trial data involving EFIRM’s use to detect in patients’ saliva the presence of mutations in the epidermal growth factor receptor (EGFR) gene, a predictive biomarker for metastatic lung cancer drug treatment (J Clin Oncol 2016, 34 suppl; 8520). The UCLA group reported almost the same results as achieved with tumor tissue DNA, the gold standard. “We are seeing a performance, a concordance, that is too good to be true,” said Wong.
Moving Beyond Proof of Concept
Though the trial produced encouraging results, it included just 37 lung cancer patients, too few to reach any definitive conclusions, especially since only six had EGFR mutations. “The authors’ results are very promising, but are only proof-of-concept promising,” said Cloud Paweletz, PhD, head of the Translational Research Laboratory at the Dana-Farber Cancer Institute’s Belfer Center for Applied Cancer Science in Boston. “Prospective validation based on the authors’ initial results are needed.”
The UCLA group envisions a broad use for saliva-based liquid biopsies in the early detection of cancer, including pancreatic cancer, but chose the lung cancer predictive biomarker mutations to establish proof of principle for EFIRM. Thoracic oncologists typically identify these lung cancer mutations from tumor tissue obtained from a lung biopsy, but because a biopsy isn’t always an option, mutations can be identified from plasma. EFIRM has the potential to be a point-of-care alternative to laboratory-based testing, since it can generate results within 15 minutes, according to Wong.
EFIRM in Detail
EFIRM, invented by UCLA bioengineer and adjunct assistant professor Fang Wei, PhD, while working on her doctoral dissertation at Peking University, uses oscillating electric fields to bring macromolecules into contact with an array of conducting polymer electrodes decorated with capture probes. The charged surface draws down macromolecules with a net positive charge, Wong explained, and reversing the field polarity repulses nonspecific sequences. The capture probes can be designed for nucleic acids or proteins.
For tumor DNA, separate detector probes, labeled with fluorescein isothiocyanate, mix with the saliva sample after the latter has been centrifuged to remove cells and stabilized with RNAse. Then, anti-fluorescein antibody conjugated to horseradish peroxidase binds the detector probes, horseradish peroxidase substrate is added, and the amperometric signal generated by the subsequent redox reaction is measured. EFIRM requires just a 40 µL sample, according to Wong. Unlike polymerase chain reaction (PCR)-based assays, he stressed, “This is direct detection.”
EFIRM is not the only device being developed for saliva diagnostics for systemic diseases. Investigators at the University of Kentucky College of Dentistry’s Center for Oral Health Research have used a device that contains a modular and miniaturized sensor system and universal analyzer, now being further refined by John McDevitt, PhD, chair of biomaterials and biomimetics at New York University College of Dentistry. They also are experimenting with a point-of-care lateral flow device. Jeffrey Ebersole, PhD, professor and associate dean for research and graduate studies at Kentucky, thinks UCLA’s EFIRM could also be broadly useful. “There’s no reason that it could not be used to look at some of the same molecules that we’re looking at in other diseases,” he said.
Challenges Ahead
Wong hopes ultimately to gain Food and Drug Administration approval for EFIRM. In two proof-of-principle studies in lung cancer—the aforementioned investigation involving 37 patients, and another with 40—Wong and his team compared in a blinded fashion EFIRM-analyzed saliva and plasma samples with tumor tissue DNA analyzed via PCR, and found excellent concordance. In both trials, there were a few cases in which the electric current values for wild-type tumors overlapped with the electric current generated by mutant tumor DNA, so EFIRM wasn’t perfect. Wong indicated that an improved EFIRM is now “robust for clinical performance.”
Strictly speaking, the researchers couldn’t report a false positive or false negative rate, because they hadn’t established a cutoff to define the presence of these mutations—the mutation signal is treated as a continuous variable, a challenge that will need to be remedied in order for EFIRM to advance. “In the diagnostic setting you can’t look at a whole population of people and create a new cutoff; you have to have the cutoff in advance for an individual sample,” said University of Pittsburgh thoracic oncologist James Herman, MD. Wong acknowledged this. The cutoff “needs to be in place by the time EFIRM turns into a clinical assay, and hopefully that will be in the next six months,” he indicated.
Wong is now preparing to analyze the serum of 300 lung cancer patients archived at NYU Langone Medical Center in New York City. But this trial will analyze blood plasma, not saliva, and will be a retrospective analysis, introducing possible selection bias. Wong said his former postdoctoral scholar, Wei Liao, PhD, will launch a large trial in China next year, sponsored by the company Liao founded, EZLifeBio. This new China trial could lead to regulatory approval there, according to Wong. Meanwhile, he is actively seeking a sponsor for future trials in North America.
Even as Wong and his colleagues work to advance EFIRM towards clinical practice, Herman noted that as a liquid biopsy EFIRM’s current design does not detect the most clinically useful EGFR mutations in lung cancer. As now configured, EFIRM tests the exon 19 and L858R mutations, which predict response to the EGFR inhibitors, gefitinib and erlotinib, in metastatic non-small cell lung carcinoma. But virtually all these patients already get a lung biopsy—it’s required for diagnosis—and the tumor tissue gets tested for EGFR mutations. Occasionally a patient presents with metastatic disease so obvious that a tissue biopsy isn’t necessary, and a liquid biopsy might be useful. “That specific application would be rather small,” said Herman. “The real place where people are looking at liquid biopsies is in the progressive setting, when therapeutic resistance occurs.” One EGFR mutation, T790M, accounts for most of these drug-resistant cases. UCLA is now optimizing EFIRM for the T790M mutation, according to Wong. “It will be important to take the device to the next level,” he said.
As Wong and his colleagues continue developing EFIRM as a tool in lung cancer diagnostics, they are also working on a separate line of investigation involving pancreatic ductal adenocarcinoma (PDAC). The team recently reported creating EFIRM probes for the top four KRAS mutations (G12D, G12V, G12R, and G12C with > 90% frequency in total) associated with PDAC (J Clin Oncol 2016, 34 suppl; e15702).
Still, Wong acknowledged that EFIRM has a long way to go to make its mark in clinical practice. As he put it, “Hopefully these efforts…could reach a point that could catalyze a momentum.”
Author: Ken Garber. He is a freelance writer in Ann Arbor, Michigan. Email: kengarber@prodigy.net
Source: AACC
New method measures the risk of type 2 diabetes in blood
“This could motivate a person at risk to change their lifestyle”, says Karl Bacos, researcher in epigenetics at Lund University.
Predicting the onset of diabetes is already possible by measuring the blood glucose level average, HbA1C, over time. However, the predictive potential of this method is modest and new methods are needed.
The discoveries made by the research group at Lund University have now made it possible to measure the presence of so-called DNA methylations in four specific genes, and thereby predict who is at risk of developing type 2 diabetes, long before the disease occurs. Methylations are chemical changes that control gene activity, that is, whether they are active or not.
“The hope is that this will be developed into a better way to predict the disease”, says Karl Bacos, first author of the study.
The researchers started by studying insulin-producing beta cells from deceased persons. They found that the DNA methylations in the four genes in question increased, depending on the donor’s age. This in turn affected the activity of the genes.
When these changes were copied in cultured beta cells, they proved to have a positive effect on insulin secretion.
“We could then see the same DNA methylation changes in the blood which was really cool”, says Karl Bacos.
The blood samples from the participants of two separate research projects – one Danish and one Finnish – were then studied and compared with blood samples taken from the same participants ten years later. The Finnish participants, who had exhibited higher levels of DNA methylation in their first sample, had a lower risk of type 2 diabetes ten years later. In the Danish participants, higher DNA methylation in their first sample was associated with higher insulin secretion ten years later. All of the Danish participants were healthy on both occasions, whereas approximately one-third of the Finnish participants had developed type 2 diabetes.
“Increased insulin secretion actually protects against type 2 diabetes. It could be the body’s way of protecting itself when other tissue becomes resistant to insulin, which often happens as we get older”, says professor and research project manager Charlotte Ling.
The studies were based on a relatively small number of participants, and a selection of genes. The researchers therefore now want to continue with finding markers with a stronger predictive potential by implementing so-called epigenetic whole-genome sequencing when analysing a person’s entire genetic make-up and all the DNA methylations that come with it, in a larger population group.
The research group has previously shown that age, diet and exercise affect the so-called epigenetic risk of type 2 diabetes.
“You cannot change your genes and the risks that they entail, but epigenetics means that you can affect the DNA methylations, and thereby gene activity, through lifestyle choices”, says Charlotte Ling.
Source: Lund University
1st World Sepsis Congress: completely online and free
The 1st World Sepsis Congress will take place completely online on September 8th and 9th, 2016, as a prelude and introduction to the fifth World Sepsis Day on September 13th, 2016.
Participation is open to everyone with an internet connection and free of charge.
In 13 distinctive sessions, over 70 speakers from over 20 countries will give 10-minute keynotes and presentations on the number one preventable cause of death worldwide: sepsis. After each talk, the speakers will answer live questions from the audience.
Highlights include the Opening Session, titled “Sepsis – A Global Health Threat”, with German Minister Helge Braun, Marie-Paule Kieny, Assistant Director-General of the WHO, Achim Steiner, former Under-Secretary General UN and Director of UNEP, and Joe Kiani, Founder & CEO of the Patient Safety Movement Foundation.
No more information is available on infobioquimica.org. For further requests, you can contact the organizers of the event.
Uric acid, gout and kidney disease: The chicken or the egg?
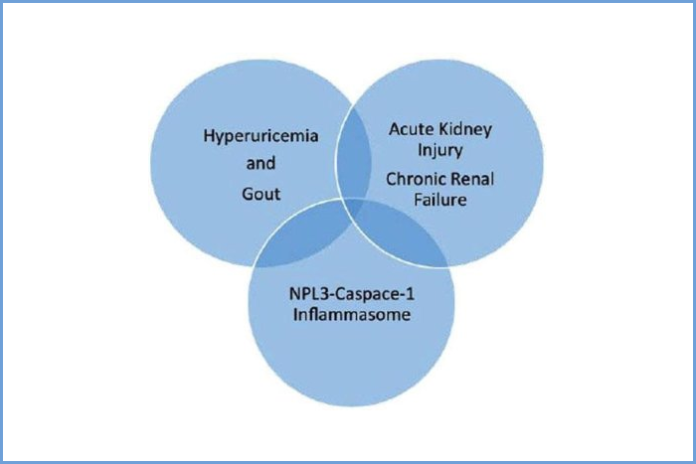
The increasing prevalence of both gout and chronic kidney disease has led to a growing interest in the association between hyperuricemia (an abnormally high level of uric acid in the blood) and kidney disease.
A new thematic issue of The Open Urology & Nephrology Journal, titled ‘Current Perspectives in Hyperuricemia, Gout and the Kidney,’ reports on the interplay of various factors, particularly the role of the kidney in uric acid excretion on the one hand, and the possible impact of hyperuricemia on progression of renal disease on the other. The common patho-physiological link appears to be the chronic, low-grade, systemic inflammation that is intrinsic to both conditions, and that may explain some of the perplexing observations noted in these clinical conditions.
This thematic issue discusses the effect of the activation of the innate immune system, through stimulation of the NLRP3 inflammasome, leading to the subsequent generation of interleukins and the release of cytokines and chemokines, and how these factors interact in the complex interplay between hyperuricemia, gout and kidney disease.
Additionally, with the recent updates in clinical management guidelines for acute and chronic gout, and given that there are special considerations in specific patient populations, articles in the issue incorporate recommendations from three different medical perspectives: the primary care physician, the rheumatologist and the nephrologist.
Source: ScienceDaily
IFCC’s Forthcoming Congresses – August Issue
Calendar of IFCC Congresses/Conferences and Regional Federation’s Congresses
| Nov 26 – 29, 2016 | 14th Asia-Pacific Federation for Clinical Biochemistry and Laboratory Medicine Congress | Taipei, TW |
| Jun 11 – 15, 2017 | IFCC-EFLM EuroMedLab 2017 | Athens, GR |
| Sep 17 – 22, 2017 | XXIII COLABLIOCLI Congress 2017 and XI Uruguayan Congress of Clinical Biochemistry | Punta del Este, UY |
|
Oct 20 – 22, 2017 |
Durban, ZA | |
| Oct 22 – 25, 2017 | XXIII IFCC WorldLab 2017 | Durban, ZA |
| May 18 – 23, 2019 | IFCC-EFLM EuroMedLab 2019 | Barcelona, ES |
| May 24 – 28, 2020 | XXIV IFCC WorldLab 2020 Seoul | Seoul, KR |